|
Chapter 1 Thesis introduction Scientists have been, and are still, intrigued by the amazing secondary sexual characteristics with which some animals are equipped. The evolution of these traits and their associated behaviours can be explained by the theory of sexual selection, first proposed by Darwin in 1871. Sexual selection is based on the differential reproductive success of individuals, and involves processes like mate choice and competition for mates. Contrary to natural selection, extreme features that can hinder survival of the animal are often selected for. Well known examples are the extravagant tail feathers of birds (Møller, 1998), the impressive antlers of ungulates (Clutton-Brock, 1981), and the large nuptial gifts of insects (Proctor et al., 1995).One behaviour that remains to be explained by the theory of sexual selection is the bizarre dart shooting that is seen in several species of terrestrial snails (Baur, 1998). This courtship behaviour consists of each hermaphrodite mating partner forcefully stabbing a so-called "love dart" through the skin of the mating partner, where it usually stays lodged (Figure 1). Despite the injury, mating partners show only a small avoidance response and continue their mating behaviour. In this thesis, I attempt to understand both the function of dart shooting and the means by which dart shooting is expressed by the central nervous system. Therefore, my thesis research on the love dart called for a variety of approaches, including behavioural, physiological, game theoretical, and neurophysiological. In the first part of this introductory chapter, I give an overview of mating behaviour and dart shooting in snails with special reference to the studied species, the garden snail Helix aspersa. I also discuss the hypotheses that have been proposed to explain dart shooting behaviour, and I formulate the first central question dealt with in Chapters 2, 3, and 4: What is the function of dart shooting? In the second part of this chapter, I focus on the neurobiological aspects of dart shooting and mating behaviour in snails, and I formulate the second central question dealt with in Chapter 6: How are dart shooting and the other components of mating behaviour controlled by the central nervous system? Behavioural aspects of dart shooting and mating Courtship and copulation in snails Dart shooting behaviour constitutes only part of an elaborate mating sequence, therefore, I shall also include other components of the mating behaviour. Dart shooting is exclusive to the Stylommatophora, an order of pulmonate land snails characterised by simultaneous hermaphroditism. In such hermaphrodites each mating partner fulfils both the male role and the female role during a given mating encounter. Adamo and Chase (1988) described the mating behaviour of Helix aspersa in detail. This snail's mating behaviour consists of three phases: introduction, dart shooting, and copulation. At the start of introduction two potential mating partners meet and initiate tentacle contacts and lip contacts. During these behaviours, the genital atrium - which is normally internal - is slowly everted through the genital pore and becomes visible as a white bulge on the right side of the head. This is referred to as the genital eversion and exposes the female and male genital openings, but leaves the penis inside the animal. The animals position themselves head-on and occasionally interrupt courtship to crawl in a circle or several semicircles to reposition themselves with regard to the partner (circling behaviour: Adamo & Chase, 1988). In the advanced stages of genital eversion, which are numbered 1 to 5 based on their form and increasing size (Adamo & Chase, 1988), animals will often make lip-genital contacts and occasionally bite each other. When the higher levels of sexual excitement are reached the animals often push their genital eversions together. These behaviours are performed during half an hour on average, although they can continue for up to one hour (Adamo & Chase, 1988). The introductory phase ends when one of the mating partners shoots its love dart. The term "love dart" is used because of the analogy with Cupid's arrow (reviewed by Kothbauer, 1988). The dart, however, does not actually fly through the air as the term "dart shooting" implies. Instead, it is expelled by a forceful eversion of the muscular dart sac, from the genital eversion, when the mating partners are in close bodily contact (Adamo & Chase, 1988). Normally, the dart detaches from the dart sac when it pierces through the partner's skin (Figure 1), therefore, it remains lodged in the recipient and can be internalised into the body cavity (Adamo & Chase, 1990). Usually, not long after one individual of the mating pair has shot its dart, the partner will do the same (average interval eight minutes; Adamo & Chase, 1988).
Once a snail has shot its dart it will attempt to copulate by everting its penis through the male pore of the genital eversion (stage 6 eversion: Adamo & Chase, 1988) and try to intromit into the female genital opening of the partner. Successful copulation may take multiple attempts because the penes of both animal have to be inserted simultaneously, and an individual will not allow penis intromission until it has reached the stage of penis eversion (Chung, 1987). Once simultaneous intromission is achieved the snails become inactive (Figure 1), partly withdraw their tentacles, and remain in this copulatory position for several hours (average 422 min; Adamo & Chase, 1988). During this period spermatophores are formed and transferred, one by each mating partner. An overview of the morphological structures described below is given in Figure 1 of Chapter 3 (page 33). The spermatophore is formed in the penial complex and consists of four parts: The head, the neck, the body and the tail. The empty head and neck of the spermatophore are probably formed in the penis; the body of the spermatophore is formed in the epiphallus and envelopes the sperm; the tail of the spermatophore is produced by the flagellum (Lind, 1973). After formation, the spermatophore is transferred into the bursa tract diverticulum of the partner. This organ forms part of the bursa complex, which consists of the bursa tract diverticulum and the bursa copulatrix with its accompanying bursa tract. Sperm swim through the tail of the spermatophore to reach the spermoviduct via the genital atrium (Lind, 1973). Only a small percentage (0.1 % in Helix pomatia: Lind, 1973) eventually reach the sperm storage organ, the spermatheca, at the end of the spermoviduct. The vast majority of the sperm are instead transported through muscular actions - together with the spermatophore - to the bursa copulatrix where they are digested (Lind, 1973). For Helix pomatia it has been shown that digestive secretions from the bursa copulatrix enter the spermatophore after copulation (Hryniewiecka-Szyfter & Redziniak, 1976). However, in that species the spermatophore is received directly in the bursa tract. Whether such digestive enzymes also enter the spermatophore in the bursa tract diverticulum of Helix aspersa is not known. The sperm that escape digestion and reach the spermatheca can be stored in that organ for over a year before being used for the fertilisation of eggs (Tompa, 1984). The storage of received sperm is necessary because egg laying does not immediately follow copulation. Moreover, several matings with different partners can take place before eggs are fertilised and laid. The eggs of Helix aspersa, as those of many terrestrial snail species, are provided with calcium in the shell (Tompa, 1984). In Helix aspersa the average egg clutch contains 50 to 100 eggs (Daguzan, 1981). Hence, a large amount of calcium is required for egg laying (Tompa & Wilbur, 1977). The love dart of Helix aspersa The nine-millimetre long love dart (see Figure 2) is produced and stored in a special organ, the dart sac (Hunt, 1979). The production or regeneration of a dart takes approximately six days (Tompa, 1982), and production seems to be initiated only after eversion of the dart sac during the first mating (Chung, 1986a). The initiation of dart formation might be triggered by the loss of the gelatinous substance present in the dart sac of virgins (Chung 1986a). The dart is composed almost completely of calcium carbonate (CaCO3) in the form of micro-crystalline aragonite (Helix pomatia: Hunt, 1979; Helix aspersa: Dillaman, 1981). The calcareous structure is protected by a protein sheath - probably also produced by the dart sac - which represents about 5 to 6 % of the total weight (Hunt, 1979). The dart sac is directly connected to the digitiform glands that secrete a mucus onto the dart immediately before it is shot (Chung, 1986b; Adamo & Chase, 1990).
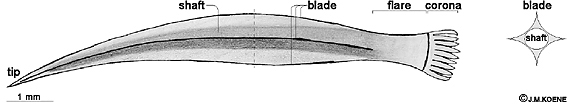
The shape of the dart is species-specific and is often used by malacologists to distinguish between closely related species (Tompa, 1984). Therefore, Webb (1951) proposed that darts might be involved in species recognition, but this idea found little support. The dart of Helix aspersa (Figure 2) consists of a hollow shaft with four blades that are positioned perpendicular to the shaft. Hence, in cross section the dart forms a symmetrical structure resembling a Celtic cross (Hunt, 1979). The shaft is slightly curved and ends in a fine, sharp tip. The blades run along most of the length of the dart, and only disappear near the basal end. This end is called the flare and continues in the corona, which attaches the whole structure to the tubercle at the posterior end of the dart sac. This attachment is usually broken during dart shooting leaving the dart lodged in its target. Why do snails shoot darts? The bizarre phenomenon of dart shooting has still not been satisfyingly explained although scientists have pondered on it since the 17th century, when Swammerdam described the dart as an "alkaline little bone"; he suspected that it had something to do with mating (Swammerdam, 1737). Near the end of the 17th century the term love dart ("flèche d'amour") came into use (reviewed by Kothbauer, 1988). More than a hundred years later, when Ashford (1883) wrote his review of British dart shooting species, little more was known about the function of darts. Ashford expressed the general consensus of the time that the puncture caused by the dart somehow excites the mating partner. The hypothesis that sexual excitement is increased by the mechanical stimulation of the dart was first tested by Goddard (1962). He reported that pinching the skin near the genital pore increased the tonus of the penial muscles. Both Jeppesen (1976) and Lind (1976), however, were unable to confirm Goddard's findings based on observations of the mating behaviour of Helix pomatia. Instead, Jeppesen suggested that the dart might mechanically stimulate the recipient's female opening and Lind speculated that the dart serves to test the level of sexual excitement of the partner. Giusti and Lepri (1980) came to the same conclusion as Lind for several related snail species. If the dart serves to increase or test the excitement of the partner, it is expected to be used during every mating encounter. Inconsistent with this idea is the fact that the dart is deciduous and can be used once every six days (the dart regeneration time), while mating occurs more frequently (Tompa, 1984). Webb (1952) suggested that the mechanical stimulation associated with dart shooting serves to coerce the partner to co-operate in the mating process. Elaborating upon this hypothesis, Leonard (1992) proposed that the dart's purpose is to induce the partner to donate sperm. She based her argument on a sperm-trading model that she had developed earlier (Leonard, 1990), in which she assumed that the female role is preferred in internally fertilising species because of the higher assurance of reproductive success associated with this role. This preference leads to a conflict because each individual will only want to assume the preferred role (Leonard, 1990). Leonard argued that reciprocal mating - i.e. each individual fulfils the male and female role with the same partner either simultaneously or sequentially - has evolved as a solution to this conflict (1990). She suggested that the dart would have evolved to indicate the honest intention of the shooter to fulfil the less preferred male role, which would encourage the partner to reciprocate (Leonard, 1992). In the application of her model to dart shooting, however, she fails to take into account that dart shooting snails are simultaneously reciprocal hermaphrodites, i.e. they mate in both the male and the female role at the same time. Because, these snails donate and receive sperm in every mating encounter, there seems no need to stimulate the partner to fulfil its male role, which suggests that neither sexual role is more advantageous. Greeff and Michiels (1999) argued that this should be the case because in hermaphrodites both sexual roles necessarily have, on average, equal reproductive successes. The hypothesis that the dart could be a gift of calcium was first proposed by Charnov (1979), drawing on insect biology. In insects the donation of valuable nutrients along with the sperm is commonplace. For example, the male arctiid moth Utetheisa ornatrix donates an alkaloid - contained in the spermatophore - to the female that protects her, as well as the eggs she supplies with it, against predators (Dussourd et al., 1991). Likewise, the male notodontid moth Gluphisia septentrionis transfers a large amount of sodium to the female. This sodium is put into the eggs as a supplement to the sodium-poor diet of the larvae (Smedley & Eisner, 1996). Similarly, in snails the dart could be a nuptial gift of calcium because it is made almost entirely of this element (Hunt, 1979; Dillaman, 1981). Calcium is an important element for growth (Crowell, 1973) and egg laying (Tompa & Wilbur, 1977) in snails. Along the same lines, egg laying could be induced through the mechanical stimulation caused by the dart (Tompa, 1980), or even by the calcium of the dart. Tompa and Wilbur (1977) have reported that the calcium level in the blood shows a large increase right before egg laying. It is conceivable that this increase of calcium in the blood is triggered by the calcium of the dissolving dart, thus evoking egg laying. The calcium hypothesis remains a possible explanation for dart shooting, but it has never been tested. While the above suggests that the dart might be a gift of calcium, another set of hypotheses is based on the idea that the dart serves as a vehicle to introduce a bioactive substance into the recipient. The dart is covered with mucus from the digitiform glands. In 1925, by injecting digitiform gland mucus into snails, Dorello observed that it caused the blood to coagulate around the injured skin area. He also found that it contracted the skin and the penis, and reported that it seemed to bring the animal into a state of excitement. Dorello referred to this active substance as digitana because it is produced in the digitiform glands. Unfortunately, Dorello's injection experiments did not control for the mechanical stimulus of piercing the skin. Nonetheless, Börnchen (1967) supported the idea that the activity of internal organs could be altered by the introduction of mucus in the snail's haemolymph. Börnchen specifically looked at the effect of the digitiform gland mucus on the isolated heart. He observed an increase in the beating frequency and interpreted this finding as a confirmation of the increase in the overall sexual excitement as reported by Dorello (1925). The hypothesis that the dart introduces mucus into the recipient and increases excitement has been further tested by Chung (1986b) and Adamo and Chase (1990). In these studies it was found that when extracts of the digitiform glands were introduced into the haemolymph of snails the level of genital eversion was increased. Both studies controlled for the mechanical stimulation of the injection. Adamo and Chase (1990) also confirmed that the mucus on the dart enters the haemolymph of the recipient during natural dart shootings. However, the "pheromonal" effect on sexual arousal was relatively small. Mucus injection decreased mating time by only approximately 6.7 %, affected only animals with a stage 2 or 3 genital eversion, and was only marginally significant (P = 0.052, N = 17: Adamo & Chase, 1990). Adamo and Chase argued that a decrease in mating time might be significant in making the snails less vulnerable to predation (1988), although Pollard's field study on Helix pomatia (1975) indicates that snails are not more likely to be predated upon when they are mating. In a later publication, Adamo and Chase (1996) suggested - based on their own finding and previously proposed hypotheses (Charnov, 1979; Tompa, 1980; Chung, 1987) - that the dart might be used to either influence the use of the donated sperm or trigger oviposition. This hypothesis is also based upon the introduction of a biochemical substance by the dart. Hormonal factors in the dart's mucus, possibly the same hormones that are normally used to control the snail's internal processes related to reproduction (Adamo & Chase, 1996), would directly influence the partner's reproductive system. Along the same lines, Chung (1987) suggested - based on insect biology - that the dart could reduce subsequent mating by the recipient, suppress allosperm digestion, displace previously stored sperm, or prevent subsequent sperm storage. In all these cases, the dart would have a function in intrasexual selection: the competition among individuals for access to, and fertilisation of, mates. Adamo and Chase (1996) favoured the idea that the dart serves as a male instrument to manipulate the female function. However, they did not rule out the possibility that the dart evolved due to intersexual selection: the choices made by individuals, based on certain traits, for mating partners. In this hypothesis, if snails choose their partners based on dart shooting, the preference for dart shooters could cause a Fisherian runaway process. Fisher's runaway selection theory (1930) assumes that a preference exists for an arbitrary trait, and that therefore such a preferred trait confers a reproductive advantage. The result is a runaway process in which the preferred trait (dart shooting) and the preference become exaggerated. This process can lead to the development of extreme traits as the famous example of the peacock's tail. Alternatively, Charnov (1979) had suggested that dart shooting could be a demonstration of an individual's ability to metabolise and use calcium. Such a sexual signal would also have evolved through intersexual selection, and could be explained with the "good genes" hypothesis (Andersson, 1994), also known as the "handicap principle" (Zahavi, 1975). For such a sexual signal to evolve the trait (dart shooting) has to correlate with the animal's physiological (e.g. calcium) condition (Zahavi, 1975). Only individuals able to show or develop the trait demonstrate their superior quality and will be chosen as mates. In summary, three hypotheses remain able to provide an adaptive explanation for the phenomenon of dart shooting. First, the calcareous dart could function as a nuptial gift to the mating partner for the investment in eggs: the nuptial gift hypothesis (Charnov, 1979). Second, the dart could transfer a bioactive substance into the blood of the mating partner where it causes some effect on the reproductive system that enhances the shooter's reproductive success: the mate manipulation hypothesis (Adamo & Chase, 1996). Third, the dart could indicate the shooter's quality and serve as a sexual signal for the mating partner: the mate choice hypothesis (Charnov, 1979). I shall deal with these three hypotheses in Chapters 2, 3, and 4, respectively. Neurobiological aspects of dart shooting and mating The central nervous system of Helix aspersa The garden snail, as many other molluscs, has a relatively simple nervous system with large, individually identifiable neurones that control a limited behavioural repertoire. These unique features greatly facilitate studying how the central nervous system works, and molluscs have therefore become a model system for many neurobiologists interested in understanding the basic principles of the brain (Kandel, 1976). The central nervous system of the garden snail (Figure 3) consists of several ganglia that communicate though connectives. The bilateral cerebral ganglia are located above the oesophagus, while the remaining ganglia are suboesophageal. The nerves that connect the cerebral ganglia to the pleural and pedal ganglia of the suboesophageal ganglia ring are called, respectively, the cerebro-pleural and cerebro-pedal connectives. The pleural and pedal ganglia connect to the parietal ganglia, which in turn connect to the visceral ganglion that closes the suboesophageal ganglia ring. The cerebral ganglia are interconnected through the cerebral commissure, thus forming, together with the suboesophageal ganglia, a ring around the oesophagus. The cerebral ganglion is divided into three parts: the mesocerebrum, the procerebrum and the metacerebrum (also known as the postcerebrum). No special function has yet been attributed to the metacerebrum; in the procerebrum most of the olfactory information from the tentacle nerves is received and processed (Ratté & Chase, 1997); the mesocerebrum is thought to be the central control region of sexual behaviour (Chase, 1986).
Central control of dart shooting and mating The mesocerebral neurones project to the penis and the dart sac via, respectively, the nervus penis and the nervus cutaneus pedalis primus dexter (Chase & Li, 1994). The male reproductive organs and the dart sac are located in the right side of the body cavity of the snail. As a result, the mesocerebrum is significantly larger in the right cerebral ganglion. Compared to the left, the right mesocerebral neurones are 23% more numerous, with 138 neurones on average; they are also 24% larger, with an average diameter of 76.8 m m (Chase, 1986). Evidence for the function of the mesocerebrum in mating behaviour comes from in vitro electrophysiological experiments. Chase (1986) reported that when individual mesocerebral neurones were intracellularly stimulated, in semi-intact preparations, muscular responses were evoked in the penis, the dart sac, or both. Hence, these data suggested that the mesocerebrum controls both penial eversion and dart shooting. The mesocerebrum of Helix aspersa has a morphological homologue in the pond snail Lymnaea stagnalis that is called the anterior lobe. This structure is located in the same anteromedial region of the cerebral ganglion; it shows a similar bilateral asymmetry; and its neurones also innervate the penis (Lymnaea stagnalis does not possess a dart sac). The right anterior lobe neurones cause eversion of part of the penial complex when they are electrically stimulated in vivo, i.e. in the intact animal (De Boer et al., 1997). The neuropeptide APGWamide (Ala-Pro-Gly-Trp-NH2) mediates this eversion, probably by relaxing the penial musculature (De Boer et al., 1997), and this neuropeptide is present in most of the anterior lobe neurones (Croll & Van Minnen, 1992). These data suggest that the anterior lobe of Lymnaea stagnalis and the mesocerebrum of Helix aspersa are morphologically and functionally homologous (Chase & Li, 1994). To investigate whether the mesocerebrum also contains APGWamide, Li and Chase (1995) performed an immunocytochemical study on Helix aspersa. They found that some mesocerebral neurones contained APGWamide. Most of these neurones had projections into the nerve innervating the penis. Therefore, Li and Chase (1995) suggested that APGWamide-containing neurones mediate penial eversion, as in Lymnaea stagnalis. In the same study, other mesocerebral neurones were found to contain FMRFamide (Phe-Met-Arg-Phe-NH2). Most of these neurones projected into the nerve innervating the dart sac (Li & Chase, 1995). Because the Stylommatophora are the only dart shooting molluscs, and seemed to be the only order of Gastropoda to contain FMRFamide in this region of the brain, Li and Chase (1995) proposed that dart shooting evolved accompanied by a neural system using FMRFamide to control it. The FMRFamide-containing neurones were thus thought to mediate dart shooting. Still other neurones were found to contain both APGWamide and FMRFamide (Li & Chase, 1995). These neurones were believed to represent the ones with projections into each of the nerves innervating the penis and the dart sac, and were thought to be involved in both components of the mating behaviour, as was also previously suggested by Chase (1986) based on his electrophysiological findings. To test the proposed involvement of the mesocerebral neurones in mating behaviour I use an in vivo approach (Chapter 6) that allows me to selectively stimulate and record from these neurones in the intact animal. The in vivo approach One of the major goals of neurobiology is to understand how animal behaviour is produced and modulated by the central nervous system. Such research involves the identification of the neurones in the central nervous system that are responsible for the expression of the behaviour of interest. The relatively simple molluscan nervous system has a limited number of large, individually identifiable neurones. One can take advantage of such unique features to study the central control of behaviours. The involvement of neurones in relation to certain physiological processes or behaviours can be inferred from studies of the isolated (i.e. in vitro) central nervous system that use intracellular and extracellular electrophysiological techniques. Research on the central nervous system in vitro has resulted in knowledge about the activity of neurones, their interactions with other elements of the nervous system, their connections to different organs, and their function. One example is the central role of the neurone C3 in eliciting and co-ordinating the tentacle withdrawal reflex of Helix aspersa (Prescott et al., 1997). The exact actions of these identified neurones in the intact animal, however, are often unknown and might be different from what is observed in vitro. In vitro preparations often require that at least part of the central nervous system is denervated, meaning that some of the incoming information from the periphery, via the nerves, is removed. An important technique that helps to overcome this problem is in vivo recording (Parsons et al., 1983). This technique makes it possible to selectively stimulate and record from neurones in the central nervous system of an intact animal. Therefore, this technique provides a way to directly test the predicted functions of neurones - assessed from in vitro preparations - by working with the central nervous system in its natural environment. The great advantage of in vivo preparations is that features of the nervous system's environment that may be of importance, but that are not present in the in vitro preparation, are conserved. This technical advancement has led to results that can link complex behaviours with identified individual neurones. Other techniques make it possible to identify the neuropeptides that are present in and released by neurones (e.g. Price & Greenberg, 1977), which enables one to demonstrate that the identified neuropeptides mediate the expression of the behaviour linked with these neurones. One example is the involvement of the cerebral giant cell in the feeding behaviour of Lymnaea stagnalis, with serotonin as a neurotransmitter (Yeoman et al., 1994). The unique features of the molluscan central nervous system have led to research focussing on individual neurones and attempting to understand their actions in relation to certain behaviours. However, to control complex behaviours, as mating or egg laying, more than a single neurone is required. In these cases, rather than focussing on single identified neurones, clusters of neurones working together must be identified. Previous in vivo work The egg laying behaviour of two molluscs, the pond snail Lymnaea stagnalis and the sea slug Aplysia californica, has been studied very successfully with the above mentioned methods. Therefore, I shall briefly review some of the research done on this behaviour with the purpose of illustrating how an in vivo approach can be used effectively in neuroethological studies. Based on in vitro results, egg laying behaviour was thought to be controlled by a bilateral group of neurones in the cerebral ganglia in Lymnaea stagnalis, the caudo-dorsal cells. These neurones are electrically coupled and show synchronous bursting activity in vitro (De Vlieger et al., 1980) during which they release egg laying hormone (Geraerts et al., 1985). In Aplysia californica a bilateral group of neurones in the viceral-parietal (=abdominal) ganglia with similar discharge properties also releases egg laying hormone (Kupfermann, 1967). The only evidence for the existence of a centre that controls egg laying in Helix aspersa comes from immunocytochemical work, which suggests that it might be located in the visceral or right parietal ganglion (Van Minnen et al., 1992). In vivo recordings and stimulations have confirmed that the caudo-dorsal cells of Lymnaea stagnalis and the bag cells of Aplysia californica are the neuronal clusters controlling egg laying. Both cell clusters exhibit a discharge that initiates the behaviour (Ter Maat et al., 1986; Dudek et al., 1979). To establish the behavioural importance of these identified neurones they should be necessary and sufficient (Parsons et al., 1983). Their sufficiency has been confirmed by electrically stimulating the neurones in intact animals, which resulted in egg laying behaviour (Ter Maat et al., 1989; Ter Maat & Ferguson, 1996). Their necessity has been tested by selective ablation, but only in Aplysia brasiliana. It was found that egg laying frequency severely decreased after lesioning the bag cells (Pinsker & Dudek, 1977), but it was not completely eliminated, showing that these neurones are not absolutely necessary although they play an important role. During the discharge, the caudo-dorsal cells and the bag cells release several hormones into the blood, including the egg laying hormone (respectively: Geraerts et al., 1985; Chiu et al., 1979). When injected into the blood, the purified hormones evoke egg laying behaviour (respectively: Ter Maat et al., 1989; Chiu et al., 1979). It has also been shown that, besides the central control of egg laying, neuronal feedback from the reproductive organs is necessary for co-ordinating part of the behaviour. When the neuronal information from the eggs passing through the reproductive tract is removed by denervating the reproductive tract, parts of the egg laying behaviour are omitted (Lymnaea stagnalis: Ferguson et al., 1993; Aplysia fasciata: Ter Maat & Ferguson, 1996). The above shows that by using the in vivo techniques one is able to test the predictions that are made from in vitro experiments, and the electrical activity of a small population of neurones can be directly related to a natural behaviour. Bullock (1999) argued that the micro-stimulation technique has been particularly overlooked as a powerful method in neuroethological research. Additionally, micro-injections of the neuropeptides or neurotransmitters contained in the neurones - to mimic the natural release of these substances - can evoke the behaviour (Bullock, 1999). Combining these techniques with electrophysiological recordings during naturally occurring behaviour can provide conclusive evidence of the involvement of particular neurones in a behavioural event. In Chapter 6, The in vivo recording and stimulation technique is used to test the involvement of the right mesocerebrum in mating behaviour, with special emphasis on dart shooting and penial eversion. Also, the proposed functions of APGWamide and FMRFamide are tested by introducing these neuropeptides into the blood. The results are discussed in an evolutionary context by comparing the data with those available from other molluscs. Objectives of this thesis In this thesis, I attempt to understand both the function of dart shooting and the means by which dart shooting is expressed by the central nervous system. Therefore, different research techniques are applied. In Chapter 2, I use behavioural observations and atomic absorption spectrophotometry to overturn the previous belief that the dart is a gift of calcium. In Chapter 3, I investigate the hypothesis that a biochemical substance in the mucus carried by the dart causes an effect on the recipient. I report that the mucus affects the physiology of the female reproductive system, which leads me to speculate that the dart serves to manipulate the partner to the shooter's advantage. However, the observed effects of the mucus can also be explained as mate choice by the recipient. I deal with this possibility in Chapter 4, in which I apply the evolutionary game theory to dart shooting. The developed game of darts for snails makes different predictions for the two alternative hypotheses. These predictions are subsequently tested, and the data are found to support the mate manipulation hypothesis. In Chapter 5, I briefly review the introduction of bioactive substances (in other species) that induce direct responses in the recipient's physiology, and I propose to use the term "allohormones" for such substances. Having come to a better understanding of why darts are shot, I change the focus to neuroethology in Chapter 6, in which I try to learn how dart shooting and the other mating behaviours are controlled by the central nervous system. Using electrophysiological in vivo techniques I show that the mesocerebrum is the main control centre for mating behaviour, including dart shooting and penis eversion. A comparison with similar work done in other mollusc species suggests that this brain area has an evolutionarily conserved function in distantly related molluscs. Chapter 7 is a general discussion of some of the issues brought up in the earlier chapters and a guide for future research on dart shooting and mating behaviour in snails.
References Adamo, S.A. & Chase R. 1988. Courtship and copulation in the terrestrial snail Helix aspersa. Can. J. Zool. 66: 1446-1453 Adamo, S.A. & Chase, R. 1990. The "love dart" of the snail Helix aspersa injects a pheromone that decreases courtship duration. J. Exp. Zool. 255: 80-87 Adamo, S.A. & Chase, R. 1996. Dart shooting in Helicid snails: An "honest" signal or an instrument of manipulation? J. Theor. Biol. 180: 77-80 Andersson, M. 1994. Sexual selection, Princeton University Press Ashford, C. 1883. The darts of British Helicidae. Part I. Introductory. J. Conch. 4: 69-79 Baur, B. 1998. Sperm competition in molluscs, in Sperm competition and sexual selection, eds. T.R. Birkhead & A.P. Møller, pp. 255-306, Academic Press Ltd. Börnchen, M. 1967. Untersuchungen zur Sekretion der fingerförmigen Drüssen von Helix pomatia L. Z. Zellforsch. Mikroskop. Anat. 78: 402-426 Bullock, Th. H. 1999. Neuroethology has pregnant agenda's. J. Comp. Physiol. A 185: 291-295 Charnov, E.L. 1979. Simultaneous hermaphroditism and sexual selection. Proc. Natl. Acad. Sci. USA 76: 2480-2484 Chase, R. 1986. Brain cells that command sexual behavior in the snail Helix aspersa, J. Neurobiol. 17: 669-679 Chase, R & Li, G. 1994. Mesocerebral neurons and their role in the control of mating behaviour. Neth. J. Zool. 44: 212-222 Chiu, A.Y., Hunkapiller, M.W., Heller, E., Stuart, D.K., Hood, L.E. & Strumwasser, F. 1979. Purification and primary structure of the neuropeptide egg-laying hormone of Aplysia californica. Proc. Natl. Acad. Sci. USA 76: 6656-6660 Chung, D.J.D. 1986a. Initiation of growth of the first dart in Helix aspersa Müller. J. Moll. Stud. 52: 253-255 Chung, D.J.D. 1986b. Stimulation of genital eversion in the land snail Helix aspersa by extracts of the glands of the dart apparatus. J. Exp. Zool. 238: 129-139 Chung, D.J.D. 1987. Courtship and dart shooting behavior of the land snail Helix aspersa. Veliger 30: 24-39 Clutton-Brock, T.H., 1981. The functions of antlers. Behav. 79: 108-125 Croll, R.P. & Van Minnen, J. 1992. Distribution of the peptide Ala-Pro-Gly-Trp-NH2 (APGWamide) in the nervous system and periphery of the snail Lymnaea stagnalis as revealed by immunocytochemistry and in situ hybridization. J. Comp. Neurol. 324: 567-574 Crowell, H.H. 1973. Laboratory study of calcium requirements of the brown garden snail, Helix aspersa Müller. Proc. Malacol. Soc. Lond. 40: 491-503 Daguzan, J. 1981. Contribution à l'élevage de l'escargot Petit-Gris: Helix aspersa Müller (Mollusque Gastéropode Pulmoné Stylommatophore) 1. - Reproduction et éclosion des jeunes, en bâtiment et en conditions thermohygrométrique contrôlées. Ann. Zootech. 30: 249-272 Darwin, C. 1871. The descent of man, and selection in relation to sex, Murray: London De Boer, P.A.C.M., Ter Maat, A., Pieneman, A.W., Croll, R.P., Kurokawa, M. & Jansen, R.F. 1997. Functional role of peptidergic anterior lobe neurons in male sexual behavior of the snail Lymnaea stagnalis. J. Neurophysiol. 78: 2823-2833 De Vlieger, T.A., Kits, K.S., Ter Maat, A. & Lodder, J.C. 1980. Morphology and electrophysiology of the ovulation hormone producing neuro-endocrine cells of the freshwater snail Lymnaea stagnalis (L.). J. Exp. Biol. 84: 239-271 Dillaman, R.M. 1981. Dart formation in Helix aspersa (Mollusca, Gastropoda). Zoomorph. 97: 247-261 Dorello, P. 1925. Sulla funzione della glandole digitale del gen. Helix. Atti R. Accad. Naz. Lincei Ser. 6: 47-51 Dudek, F.E., Cobbs, J.S. & Pinsker, H.M. 1979. Bag cell electrical activity underlying spontaneous egg laying in freely behaving Aplysia brasiliana. J. Neurophysiol. 42: 804-817 Dussourd, D.E., Harvis, C.A., Meinwald, J. & Eisner, T. 1991. Pheromonal advertisement of a nuptial gift by the male moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. USA 88: 9224-9227 Ferguson, G.P., Pieneman, A.W., Jansen, R.T. & Ter Maat, A. 1993. Neuronal feedback in egg-laying behaviour of the pond snail Lymnaea stagnalis. J. Exp. Biol. 178: 251-259 Fisher, R.A. 1930. The genetical theory of natural selection, Clarendon Press: London Geraerts, W.P.M., Vreugdenhil, E, Ebberink, R.H.M. & Hogenes, Th.M. 1985. Synthesis of multiple peptides from a larger precursor in the neuroendocrine caudo-dorsal cells of Lymnaea stagnalis. Neurosci. Letters 56: 241-246 Giusti, F. & Lepri, A. 1980. Aspetti morphologici ed etologici dell'accoppiamento in alcune specie della famiglia Helicida. Atti Accad. Fisiocr. Siena XIV: 35-55 Goddard, C.K. 1962. Function of the penial apparatus of Helix aspersa Müller. Aust. J. Biol. Sci. 15: 218-232 Greeff, J.M. & Michiels, N.K. 1999. Sperm digestion and reciprocal sperm transfer can drive hermaphrodite sex allocation to equality. Am. Nat. 153: 421-430 Hryniewiecka-Szyfter, Z. & Redziniak, E. 1976. The localization of lysosomal enzymes in the bursa copulatrix of the snail Helix pomatia L. Bull. Soc. Amis Sci. Lettres Poznan, Serie D 16: 125-134 Hunt, S. 1979. The structure and composition of the love dart (gypsobelum) in Helix aspersa. Tissue & Cell 11: 51-61 Jeppesen, L.L. 1976. The control of mating behaviour in Helix pomatia L. (Gastropoda: Pulmonata). Anim. Behav. 24: 275-290 Kandel, E.R. 1976. Cellular basis of behaviour. Freeman. San Francisco Kothbauer, H. 1988. Über Liebespfeile, Schnecken und Weltbilder. Ann. Naturhist. Mus. Wien 90B: 163-169 Kupfermann, I. 1967. Stimulation of egg laying: possible neuronedocrine function of bag cells of the abdominal ganglion of Aplysia californica. Nature 216: 814-815 Leonard, J.L. 1990. The hermaphrodite's dilemma. J. Theor. Biol. 147: 361-372 Leonard, J.L. 1992. The "love-dart" in Helicid snails: a gift of calcium or a firm commitment? J. Theor. Biol. 159: 513-521 Li, G. & Chase, R. 1995. Correlation of axon projections and peptide immunoreactivity in mesocerebral neurons of the snail Helix aspersa, J. Comp. Neurol. 353: 9-17 Lind, H. 1973. The functional significance of the spermatophore and the fate of the spermatozoa in the genital tract of Helix pomatia (Gastropoda: Stylommatophora). J. Zool. Lond. 169: 39-64 Lind, H. 1976. Causal and functional organization of the mating behavior sequence in Helix pomatia. Behav. 59: 162-201 Møller, A.P. 1998. Sperm competition and sexual selection, in Sperm competition and sexual selection, eds. T.R. Birkhead & A.P. Møller, pp. 55-90, Academic Press Ltd. Parsons, D.W., Ter Maat, A. & Pinsker, H.M. 1983. Selective recording and stimulation of individual identified neurons in freely-behaving Aplysia. Science 221: 1203-1206 Pinsker, H.M. & Dudek, F.E. 1977. Bag cell control of egg laying in freely behaving Aplysia. Science 197: 490-493 Pollard, E. 1975. Aspects of the ecology of Helix pomatia L. J. Anim. Ecol. 44: 305-329 Prescott, S.A., Gill, N. & Chase, R. 1997. Neural circuit mediating tentacle withdrawal in Helix aspersa, with special reference to the competence of the motor neuron C3. J. Neurophysiol. 78: 2951-2965 Price, D.A. & Greenberg, M.J. 1977. Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusc, Prep. Biochem. 7: 261-281 Proctor, H.C., Baker, R.L. & Gwynne, D.T. 1995. Mating behaviour and spermatophore morphology: a comparative test of the female-choice hypothesis. Can. J. Zool. 73: 2010-2020 Ratté, S. & Chase, R. 1997. Morphology of interneurons in the procerebrum of the snail Helix aspersa. J. Comp. Neurol., 384, 359-372 Smedley, S.R. & Eisner, T. 1996. Sodium: A male moth's gift to the offspring. Proc. Natl. Acad. Sci. USA 93: 809-813 Swammerdam, J. 1737. The book of nature or, the history of insects translated from the original Bijbel der nature of historie der insecten by Thomas Flloyd in 1758, revised and improved by John Hill, M.D. London: C.G.Seyffert Ter Maat, A., Dijcks, F.A. & Bos, N.P.A. 1986. In vivo recording of neuroendocrine cells (caudo-dorsal cells) in the pond snail. J. Comp. Physiol. A 158: 853-859 Ter Maat, A. & Ferguson, G.P. 1996. Neuronal input contributes to sequences of Aplysia egg laying behavior. J. Comp. Physiol. A 179: 775-783 Ter Maat, A., Pieneman, A.W., Goldschmeding, J.T., Smelik, W.F.E. & Ferguson, G.P. 1989. Spontaneous and induced egg laying behavior of the pond snail, Lymnaea stagnalis. J. Comp. Physiol. A 164: 673-683 Tompa, A.S. 1980. The ultrastructure and mineralogy of the dart from Philomycus carolinianus (Pulmonata: Gastropoda) with a brief survey of the occurrence of darts in land snails. Veliger 23: 35-42 Tompa, A.S. 1982. X-ray radiographic examination of dart formation in Helix aspersa. Neth. J. Zool. 32:63-71 Tompa, A.S. 1984. Land Snails (Stylommatophora). In: The Mollusca, 7: Reproduction eds. A.S. Tompa, N.H. Verdonk & J.A.M. van den Biggelaar, pp. 47-140. Academic Press, London Tompa, A.S. & Wilbur, K.M. 1977. Calcium mobilisation during reproduction in snail Helix aspersa. Nature 270: 53-54 Van Minnen, J., Schallig, H.D.F.H. & Ramkema, M.D. 1992. Identification of putative egg-laying hormone containing neuronal systems in gastropod molluscs. Gen. Comp. Endocrin. 86: 96-102 Webb, G.R. 1951 An instance of amixia between two species of landsnails (Pulmonata, Helminthoglyptidae). Am. Nat. 85:137-139 Webb, G.R. 1952. Pulmonata, Helminthoglyptidae: sexological data on the land-snails, Cepolis maynardi & Helminthoplypta traski fiedi and their evolutionary significance. Gastropodia 1: 4-5 Yeoman, M.S., Pieneman, A.W., Ferguson, G.P., Ter Maat, A. & Benjamin, P.R. 1994. Modulatory role for the serotonergic giant cells in the feeding system of the snail Lymnaea stagnalis. I. Fine wire recordings in the intact animal and pharmacology. J. Neurophysiol. 72: 1357-1371 Zahavi, A. 1975. Mate selection - A selection for a handicap. J. Theor. Biol. 53: 205-214 |